For Which Electrode Could You Use Inactive Material
Some of the most prominent alloys and materials used as electrode materials are copper graphite titanium brass silver and platinum. H Suggest a pair of ions for the salt bridge.

Electrochem Eng L01 11 Active Electrode Vs Inert Electrode Youtube
The electrochemical performance of lithium iron phosphate LiFePO 4 electrodes has been studied to find the optimum content of inactive materials carbon black polyvinylidene difluoride PVDF polymer binder and to better understand electrode performance with variation in electrode composition.
. An electrode which has no ions in common with the solution may be called an inert electrode. Mesoscale simulations conducted by Partha Mukherjees research group at Purdue University have revealed the role of the morphology of the inactive or secondary phase material in lithium ion battery electrodes. H Suggest a pair of ions for the salt bridge.
It could be shown so far that free-standing electrode architectures can provide new lithium-insertion pathways which enhance the capability of the. F Which electrode decreases in mass during cell operation. Commonly used inactive materials Cu and Ni have high electrolyte reactivities.
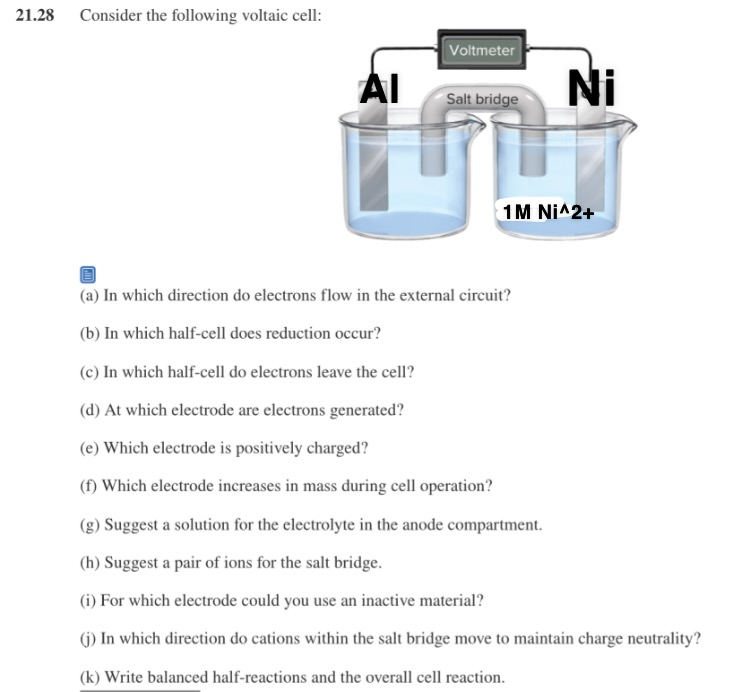
There the active electrode acts as the anode which provides the cations to the electrolytic solution. D At which electrode are electrons consumed. Consider the following voltaic celli For which electrode could you use an inactive material.
The only important point is the efficiency and economic of the electrodes. Also it is used in electroplating. A semiconductor an electrolyte a vacuum or air.
Electrodes are also used to measure conductivity. The active electrode is called active because it actively participates in the chemical reaction. Electroplating is the process where one metal is applied on another metal with the use of an electrochemical cell.
I For which electrode could you use an inactive material. The most commonly used active electrode is the copper electrode. An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit eg.
The electrophore invented by Johan Wilcke was an early version of an electrode used to study static electricity. Platinum is used as an inert electrode. The main use of electrodes is to generate electrical current and pass it through non-metal objects to basically alter them in several ways.
G Suggest a solution for the electrolyte in the cathode compartment. FREE Expert Solution Inactive Inert electrodes are electrodes which transfer electrons rather than exchanging ions with the aqueous solution in which they are immersed. Today in practice we usually use bare or coated metal electrodes.
Therefore it serves as an electron. Hello you can use Pt Rh graphite Cu and all other material for electrolysis. Active electrodes are mostly used in electroplating.
Ti and TiN were found to be the least reactive inactive materials of those tested. Electrolyte reactivity at inactive materials can contribute to cell fade and impedance. An active electrode is defined as a metal that is used in electrochemical cells.
The results published in a recent issue of ACS Applied Materials and Interfaces could be used to optimize battery performance to target application. For which electrode would you use an inactive material. Electrolyte reactivity was measured on inactive materials at the negative electrode.
Copper has better strength than. Precious metals mercury and carbon are typically used as inert electrodes. Electrical Potential and the Voltaic Cell When the switch is closed and no reaction is occurring each half-cell is in an equilibrium state.
One simple method for obtaining high-energy batteries involves reducing the amount of electrochemically inactive components in the battery eg conductive carbon binders current collectors electrolytes and separators by controlling electrode and battery design parameters eg electrode composition thickness and porosity. Inactive electrodes are usually unreactive substances such as graphite or platinum. G Suggest a solution you can use as the electrolyte in the anode compartment.
The electrodes are considered to be conductors of electric current which at the same time serves as additional material in arc welding. Apparently despite the low electronic conductivity as measured with the four-point probe for some of our electrodes with low levels of inactive material the overall cell resistance which includes the separator the lithium counter electrode the cell hardware and any contact resistances among these components for the full cells was very similar. Electrodes are used in different battery types electroplating and electrolysis welding cathodic protection membrane electrode.
The active electrode can be oxidized or reduced. Trade-offs between inactive material content and electrochemical. J In which direction do cations within the salt bridge move to maintain charge neutrality.
I For which electrode could you use an inactive material. Depending on the type of electrode used and the method of protecting the weld from oxidation arc welding can be. Copperis second only to silver in terms of bulk electrical conductivity.
Zn s Zn 2 aq 2e - in Zn metal Cu s Cu 2 aq 2e - in Cu metal Zn is a stronger reducing agent than Cu so the position of the Zn equilibrium lies. Inert electrode is an electrode that serves only as a source or sink for electrons without playing a chemical role in the electrode reaction. Some other uses include.
F Which electrode increases in mass during cell operation. These electrodes can be oxidized or reduced. Another definition would be that an unreactive metal rod placed in the solution can be called an inert electrode.
E Which electrode is negatively charged. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.
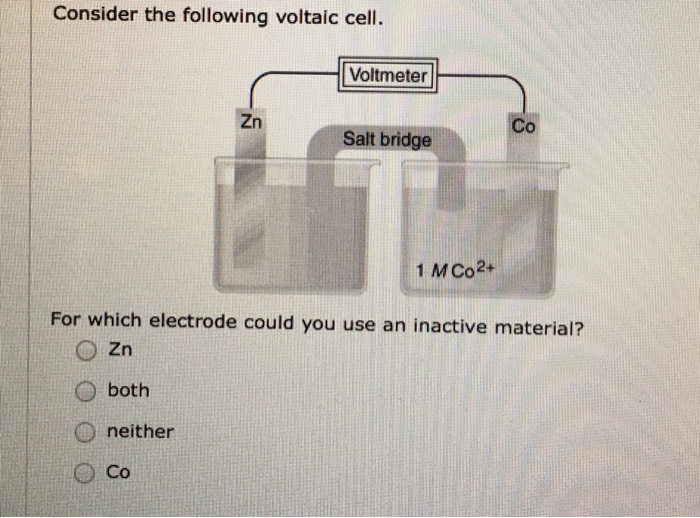
Solved Consider The Following Voltaic Cell For Which Chegg Com

Solved 21 28 Consider The Following Voltaic Cell Voltmeter Chegg Com

No comments for "For Which Electrode Could You Use Inactive Material"
Post a Comment